The Evolution and Benefits of Blow-Fill-Seal Technology in Pharmaceuticals
Blow-Fill-Seal (BFS) technology has revolutionized the pharmaceutical and healthcare industries with its advanced aseptic processing capabilities. This innovative technique involves the simultaneous formation, filling, and sealing of containers, allowing for the production of sterile packaging in a highly efficient and controlled manner.
With a rich history and continuous advancements, BFS technology has become the go-to choice for many companies seeking to ensure the highest levels of product safety and quality.
In this discussion, we will explore the different types of BFS machine technologies, the challenges of visual inspection in BFS, and the numerous benefits that come with adopting this cutting-edge approach.
Key Takeaways
- Blow-Fill-Seal technology is a widely recognized and advanced aseptic manufacturing process for forming, filling, and sealing plastic containers within a single machine.
- BFS machines, including shuttle and rotary types, offer advantages over traditional vials in terms of filling and manufacturing processes, with shuttle machines allowing for the use of insertion technology.
- BFS technology is classified as an advanced aseptic manufacturing process that minimizes product exposure and eliminates potential sources of contamination, such as component washing and operator interventions.
- Visual inspection in BFS technology is challenging due to the limited transparency of polymer containers, requiring optimized container design, proper lighting conditions, and advanced inspection equipment.
History of Blow-Fill-Seal
The history of Blow-Fill-Seal dates back over 50 years. During this time, it has become a widely recognized and advanced aseptic manufacturing process for filling sterile liquids.
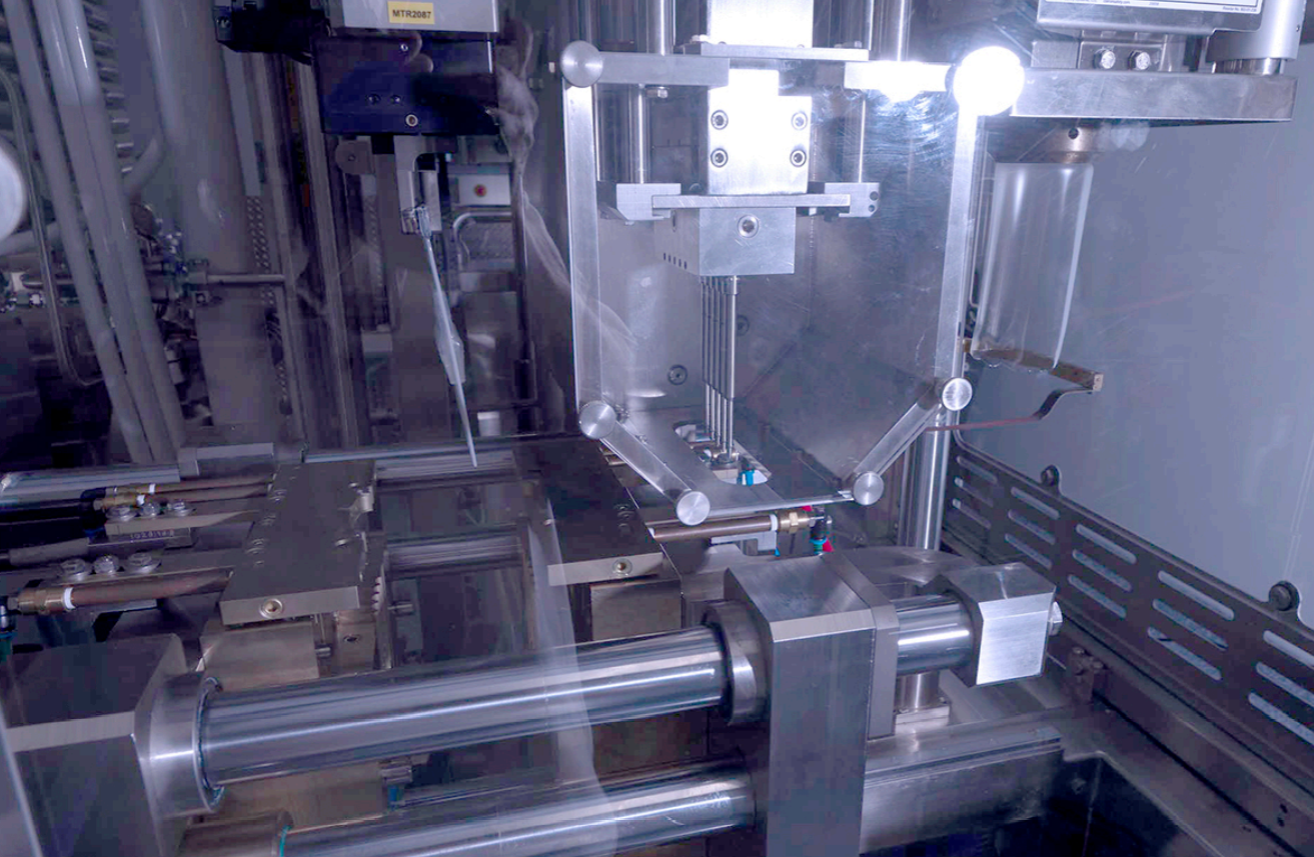
The Blow-Fill-Seal process begins with the formation of the container in a mold cavity.
After the container is formed, it is then filled with sterile liquid. The machine design ensures that the liquid is aseptically introduced into the container, maintaining its sterility.
Once the container is filled, the next step is the hermetic sealing of its opening. This sealing process ensures that the container remains airtight and the sterile liquid inside is protected from contamination.
BFS technology has proven to be highly efficient, with short product changeover times and batch-to-batch changeover times. This efficiency makes it an attractive option for pharmaceutical manufacturers.
The BFS process is widely used in the pharmaceutical industry for a variety of applications. These include the filling of ophthalmic and inhalation medicines, nasal formulations, and parenteral vaccines.
Comparison of BFS Machine Types: Shuttle vs. Rotary Technologies
BFS machine types, specifically shuttle and rotary technologies, offer distinct advantages and considerations in comparison to one another.
Shuttle machines, which were the original BFS machines, have a slower cycle time of 8-16 seconds and lower output. However, they have lower tooling costs and tooling can be quickly changed. Shuttle machines also allow for the use of insertion technology.
On the other hand, rotary machines have a faster cycle time of 3-8 seconds and can scale to larger output volumes. The entire Blow-Fill-Seal process, including forming, filling, and sealing, occurs in a single location within an enclosed chamber.
Both shuttle and rotary machines are considered advanced aseptic manufacturing processes in the pharmaceutical technology industry.
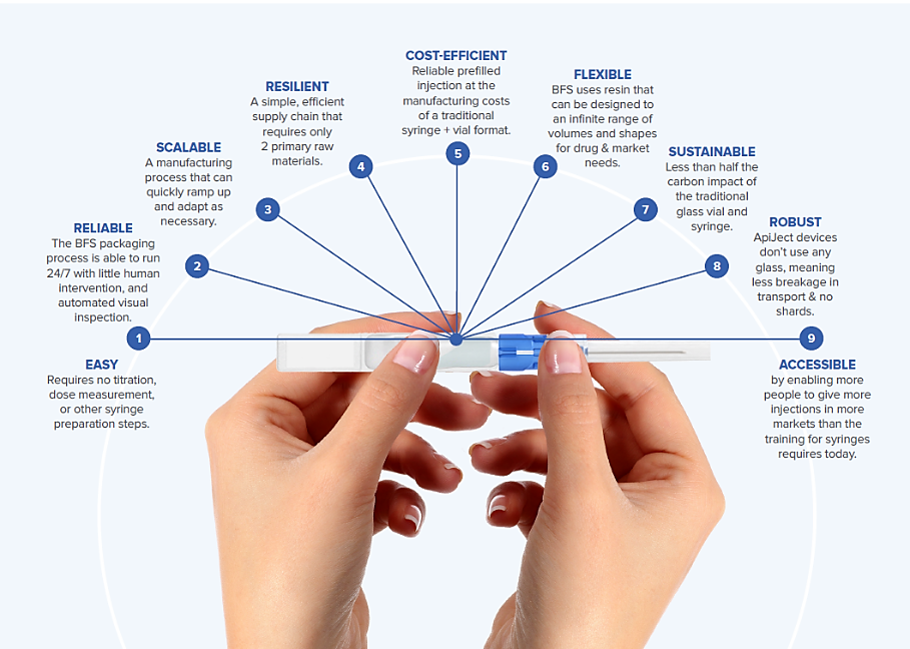
BFS machines, regardless of type, have advantages over glass vials in terms of the filling process, finish process, and packaging.
Advanced Aseptic Processing in Blow-Fill-Seal Technology
BFS Technology involves a range of features that contribute to its classification as an advanced aseptic manufacturing process.
The aseptic filling process in BFS technology is considered advanced due to its ability to minimize product exposure. The forming, filling, and sealing of the container occurs within seconds in a small critical zone, reducing the potential for contamination.
Additionally, the filling zone is inaccessible to operators during machine operation, further enhancing the aseptic nature of the process. In both BFS rotary and shuttle machines, the filling zone is within an ISO 5 environment, ensuring a controlled and sterile environment.
This aseptic liquid processing technology offers significant advantages over traditional filling processes, as it eliminates potential sources of contamination, such as component washing and operator interventions.
Visual Inspection Challenges in Blow-Fill-Seal Technology
Visual inspection poses unique challenges in Blow-Fill-Seal Technology due to the limited transparency of polymer containers compared to traditional glass vials. The partially transparent nature of the containers makes it difficult to visually inspect the contents for any potential defects or contaminants. This can be a concern, as visual inspection is an important part of the process in drug manufacturing to ensure the product is free of visible particles.
To address these challenges, manufacturers of BFS containers need to consider the following:
- Container design: Optimize the design of the polymer containers to allow for better visibility and easier inspection of the contents.
- Lighting conditions: Ensure proper lighting conditions in the inspection area to enhance visibility and detect any potential defects or contaminants.
- Inspection equipment: Invest in advanced inspection equipment that can effectively detect any abnormalities in the containers, such as particles or defects.
- Training and expertise: Provide adequate training to inspection personnel to develop their expertise in identifying potential issues during visual inspection.
Benefits of Blow-Fill-Seal Technology
The advantages of BFS technology extend beyond addressing the challenges of visual inspection in drug manufacturing, offering cost-efficiency and streamlined processes. Here's a look at the core benefits of BFS technology:
- Operational Efficiency: BFS machines are highly reliable and require minimal human intervention, leading to good efficiency usage.
- Lower Variable Costs: Unit-dose packaging with BFS can result in lower variable costs compared to single-dose glass vials or prefilled syringes.
- Lower Upfront Capital Expenditure: BFS lines typically require lower upfront capital expenditure for installation compared to traditional glass filling lines.
- Compact Design: BFS filling lines are more compact than traditional glass filling lines, saving space and reducing installation costs.
- Elimination of Washing and Dehydrogenation Tunnels: BFS machines do not require washing and dehydrogenation tunnels for vial infeed, further reducing installation costs.
- Simplified Process: BFS eliminates the need for stoppering and capping stations on the outlet side of the filler, simplifying the manufacturing process and reducing costs.
- Space Utilization: Compact BFS lines and fewer machines allow for better manufacturing space utilization.
- Reduced Qualification Requirements: With fewer machines involved in the initial installation, BFS lines have lower overall qualification requirements, leading to faster installation timelines and reduced costs.
- Lower Per Unit Manufacturing Costs: Overall, BFS offers lower per unit manufacturing costs, reduced installation costs, fewer space requirements, and faster installation timelines compared to traditional glass filling lines.
Top Questions Answered by Experts
What is the Blow-Fill-Seal technique?
The Blow-Fill-Seal technique is a method used to aseptically package liquid pharmaceuticals. It involves forming the container, filling it with the product, and sealing it in one continuous process. This technique is commonly used for single-use vials and ampoules, ensuring product sterility and integrity.