Blow-Fill-Seal: Integrating Container Formation, Filling, and Sealing in One Process
Blow-Fill-Seal (BFS) technology represents a significant advancement in aseptic packaging, combining container formation, product filling, and sealing within a highly automated and efficient single-line process. Developed over five decades ago, BFS enhances sterility assurance, substantially reduces contamination risk, and lowers manufacturing costs by eliminating multiple stages typically involved in packaging systems. Widely recognized for its operational efficiency, BFS technology can fundamentally transform production dynamics by integrating these key processes.
Key Takeaways
- Blow-Fill-Seal is an aseptic packaging method integrating container formation, filling, and sealing in one continuous process.
- This technology significantly reduces contamination risks by maintaining high sterility standards throughout production.
- BFS technology enhances production efficiency by streamlining the packaging process, reducing manual intervention, and minimizing waste.
- ApiJect's BFS container design introduces a single-dose, prefilled syringe format that enhances cold-chain efficiency and convenience.
Blow-Fill-Seal Technology
BFS packaging represents a significant advancement in the aseptic packaging of pharmaceutical and healthcare products.
Tracing its origins provides insight into how this innovation has evolved to meet stringent sterility requirements.
History of Blow-Fill-Seal Technology
Over the past five decades, the development of Blow-Fill-Seal technology has revolutionized the method of aseptic manufacturing in the pharmaceutical industry. Originating as a novel approach to packaging sterile liquids, the BFS method integrates machine design that forms, fills, and hermetically seals containers in a continuous, automated sequence.
This innovation guarantees the highest standards of sterility and efficiency which is critical for producing safe and effective pharmaceuticals. BFS supports the production of a diverse range of medical solutions, including ophthalmic medicines and parenteral vaccines.
The technology's adaptability and efficiency have made it indispensable, upholding the freedom to deliver high-quality, contamination-free healthcare products globally.
Benefits of Blow-Fill-Seal
Building on its historical evolution, the benefits of Blow-Fill-Seal technology highlight its transformative impact on pharmaceutical manufacturing efficiency and cost-effectiveness. This method excels in producing plastic containers through a streamlined aseptic filling process, greatly reducing the risk of contamination.
With its compact design, BFS technology guarantees efficient space utilization and eliminates the need for extensive washing tunnels, simplifying the production landscape. Such a streamlined process not only achieves lower variable costs but also reduces per-unit manufacturing expenses.
Moreover, the reduction in equipment and space requirements inherently lowers upfront capital expenditure. This culmination of factors makes BFS a compelling choice for pharmaceutical companies prioritizing efficiency and economic freedom in their operations.
How Does Blow-Fill-Seal Work?
BFS technology represents a highly advanced method for packaging sterile products.
This automated process integrates container formation, product filling, and sealing within a continuous, aseptic environment.
To fully appreciate its efficiency and application, we will now explore the step-by-step process involved in BFS technology.
The Step-by-Step Process
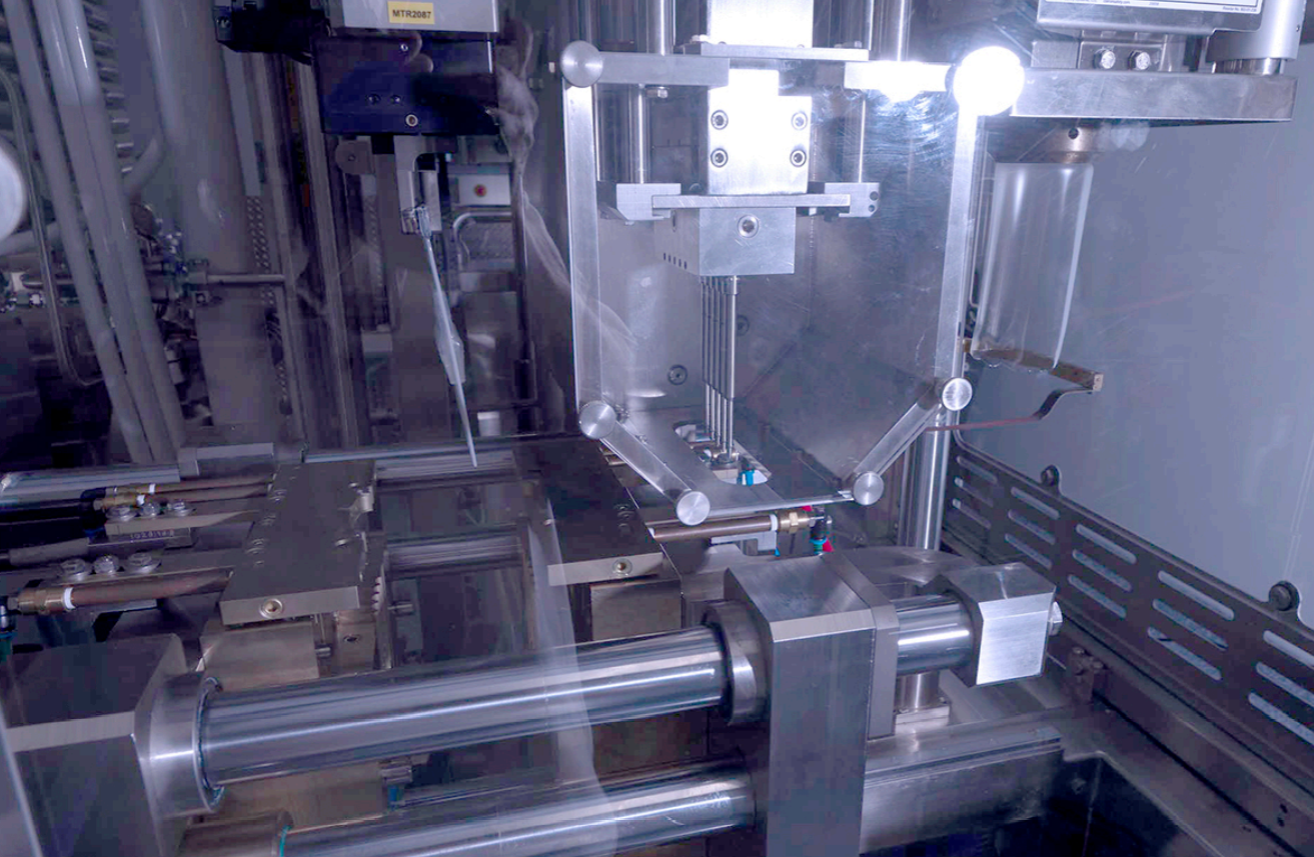
BFS technology encompasses a streamlined, three-step process: container formation, aseptic filling, and hermetic sealing, each crucial to guaranteeing the sterility and integrity of pharmaceutical products.
Initially, BFS machines heat and extrude pharmaceutical-grade plastic into a parison, which is then molded into the container form. This container formation establishes the foundation for sterile drug products.
Subsequently, the aseptic filling process begins, where a filling mandril with precise needles injects the pharmaceutical liquid into the containers under sterile conditions, maintaining container integrity.
Blow-Fill-Seal Packaging
BFS technology greatly streamlines the packaging process, especially for liquid products, by integrating blow molding, filling, and sealing in a continuous, sterile operation. This method offers substantial operational advantages over traditional glass filling lines, enhancing production efficiency and reducing contamination risks.
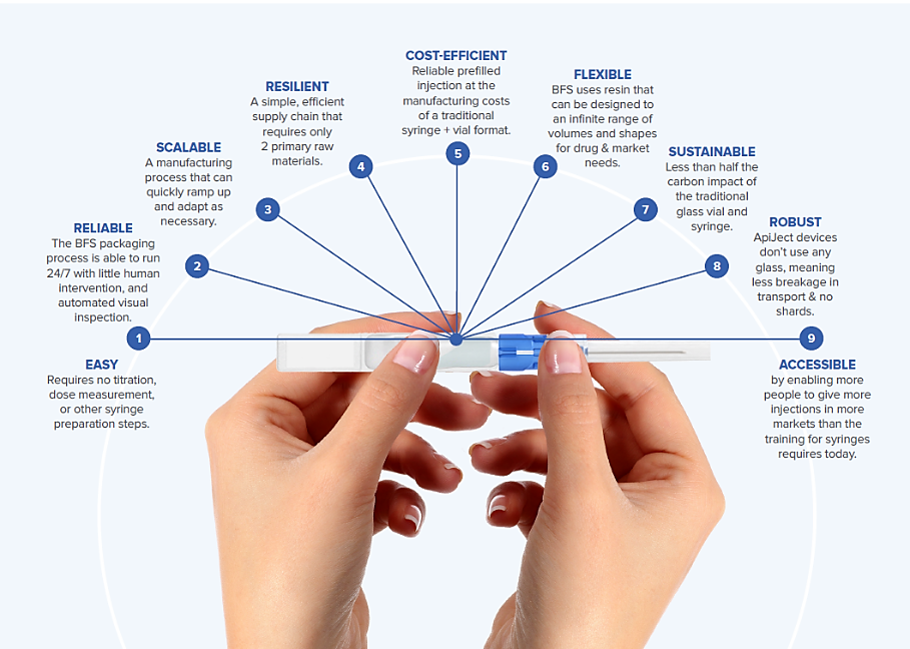
A notable innovation in this field is ApiJect's BFS container design, which introduces unique features that further optimize the safety and functionality of pharmaceutical packaging.
Blow-Fill-Seal Liquid Packaging Process
Innovative advancements in liquid packaging are epitomized by the BFS process, which integrates container formation, filling, and sealing into a continuous, automated system. This seamless manufacturing process is complemented by an engraving process for essential data such as drug product details, and a protective foil that guarantees airtight sealing for top-notch product safety.
Operational Advantages in Efficiency: Blow-Fill-Seal vs. Glass Filling Lines
Comparing operational efficiencies, BFS packaging systems outshine traditional glass filling lines when it comes to space utilization and output rates. Unlike glass container lines, BFS containers require a smaller footprint, leading to significant space savings and higher efficiency in production environments that value freedom and flexibility.
Additionally, BFS technology achieves filling efficiency rates of 90-95%, comparable to or surpassing those of glass lines. However, efficiency can be influenced by several design factors including the proximity of filling vessels to fillers, compounding batch size, tank volumes, and IPC requirements. These elements must be optimized to fully leverage the wide range of operational advantages offered by BFS technology over traditional glass filling systems.
ApiJect's Innovative BFS Container Design
Building on the operational efficiencies discussed earlier, ApiJect's BFS container design introduces a transformative approach to liquid pharmaceutical packaging. The ApiJect technology platform leverages BFS technology to produce single-dose sterile liquid-filled containers, guaranteeing aseptic fill-finish quality and enhancing transportability. These pharmaceutical-grade plastic containers exemplify the ability to rapidly address global health needs through scalable production capabilities.
ApiJect's design embodies a commitment to delivering healthcare solutions that are both practical and accessible worldwide.
Top Questions Answered by Experts
Why is BFS used?
Blow-fill-seal is a manufacturing process used to produce sterile liquid-filled containers, such as pharmaceutical vials and ampoules. This automated technique allows for the efficient and aseptic production of these containers, reducing the risk of contamination and ensuring the safety and quality of the final product.
What specific measures are in place to minimize contamination risks in BFS?
To minimize contamination risks in blow-fill-seal manufacturing, rigorous environmental monitoring and stringent cleanroom protocols are implemented. The entire process occurs in an enclosed, sterile environment to prevent microbial and particulate contamination.
Can BFS technology be easily scaled to meet the demands of large-scale pharmaceutical production?
Blow-Fill-Seal (BFS) technology can be scaled for large pharmaceutical production runs. However, careful planning and investment in specialized equipment are required to ensure quality and meet high-volume demands.
What are the cost benefits of implementing BFS technology over traditional methods, especially in terms of equipment and space?
Blow-fill-seal technology requires less equipment and floor space compared to traditional methods of manufacturing plastic containers. This all-in-one system forms, fills, and seals containers in a continuous process that eliminates the need for separate machines and reduces facility size requirements.