Reimagining Injectable Drug Delivery with the Prefilled ApiJect Injector
A delivery system designed to bring together the benefits of prefilled syringes with the manufacturing efficiency of multi-dose presentations.
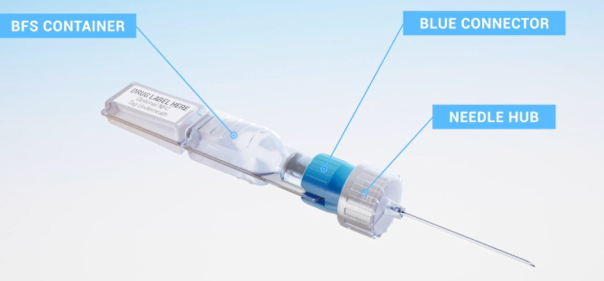

A Product of Two Trusted Technologies
We combine the manufacturing technologies of Blow-Fill-Seal (BFS) and injection molding to enable injextable medicines to be fill-finished in a prefilled format at any scale and at a lower total cost of dose delivery.

Improving Access with Time and Cost-Efficiency
The ApiJect Platform has simplicity and reliability designed into its material procurement and supply chain, requiring only two primary materials: pharmaceutical-grade resins (LDPE and PP) and cannula.

Customized to Your Products
We work with your team to design and customize the right BFS drug delivery system for your liquid drug product.
SITUATION
Amid the global COVID-19 pandemic, Fareva, a leading CDMO, recognizing supply chain vulnerabilities, partnered with ApiJect under a 10-year agreement supported by the €100 billion France Relance initiative to bolster Blow-Fill-Seal (BFS) capabilities for localized production and scalability.
SOLUTION
Fareva installed three BFS lines at Excelvision, Annonay, to achieve an annual capacity of over 500 million doses, utilizing ApiJect's technology for streamlined, high-volume fill and finish. The collaboration, supported by France Relance, emphasizes a Biosafety Level 2 environment and ApiJect's role in providing components for ready-to-use prefilled injectors.
IMPACT
The integration of three BFS lines and ApiJect's technology significantly impacts Europe's pharmaceutical manufacturing, addressing the need for increased vaccine production. Aligned with France Relance objectives, the collaboration creates an agile system, leveraging Fareva's BFS expertise and ApiJect's injector technology, with the anticipation of initiating validation batches in June 2022—a crucial step in addressing global health challenges.
SITUATION
In response to the urgent need for increased domestic fill-finish capacity for potential COVID-19 vaccines, ApiJect partnered with The Ritedose Corporation (TRC) in 2020. The collaboration, initiated under the U.S. Government's Project Jumpstart, aimed to operationalize a Single-Dose Drug Delivery Fill-Finish Facility capable of producing up to 45 million Blow-Fill-Seal (BFS) units per month within an accelerated timeline of fewer than seven months.
SOLUTION
ApiJect and TRC successfully upgraded three existing BFS lines at TRC's Columbia, South Carolina facility to bio-safety level 2 (BSL-2) conditions, aligning with the government's requirements. This involved modifying air handling systems, adding airlocks, introducing an autoclave for waste decontamination, and implementing a Biokill system. ApiJect also upgraded BFS machines and collaborated closely with TRC to align design and construction teams. The project was executed on an aggressive timeline, overcoming challenges in procuring necessary equipment such as the decon autoclave and Biokill system.
IMPACT
ApiJect met the HHS/DOD timetable for Project Jumpstart, achieving operational status for the first BFS line on October 30, 2020, and the second line a month later. This collaboration provided the U.S. Government with a domestic surge capacity, enabling the fill-finish of 45 million BFS containers per month with sterile liquid pharmaceuticals within a BSL-2 environment. The successful execution of the project within the accelerated timeline stands as a testament to the collaborative efforts between ApiJect and TRC, showcasing the effectiveness of their partnership under challenging circumstances.
Discover the ApiJect Platform
Why Prefilled Plastic Injectables are the Future
Cost-Effective Manufacturing
Our prefilled plastic injectables cut both downstream and upstream manufacturing expenses by minimizing breakage, overfill, cold chain storage, and shipping costs. Unlike glass syringes and single-dose vials, which demand rigorous quality checks, sterilization, and meticulous handling, our plastic injectables prioritize efficiency. This streamlined production process drastically reduces labor and quality control costs, making healthcare more affordable for all.




